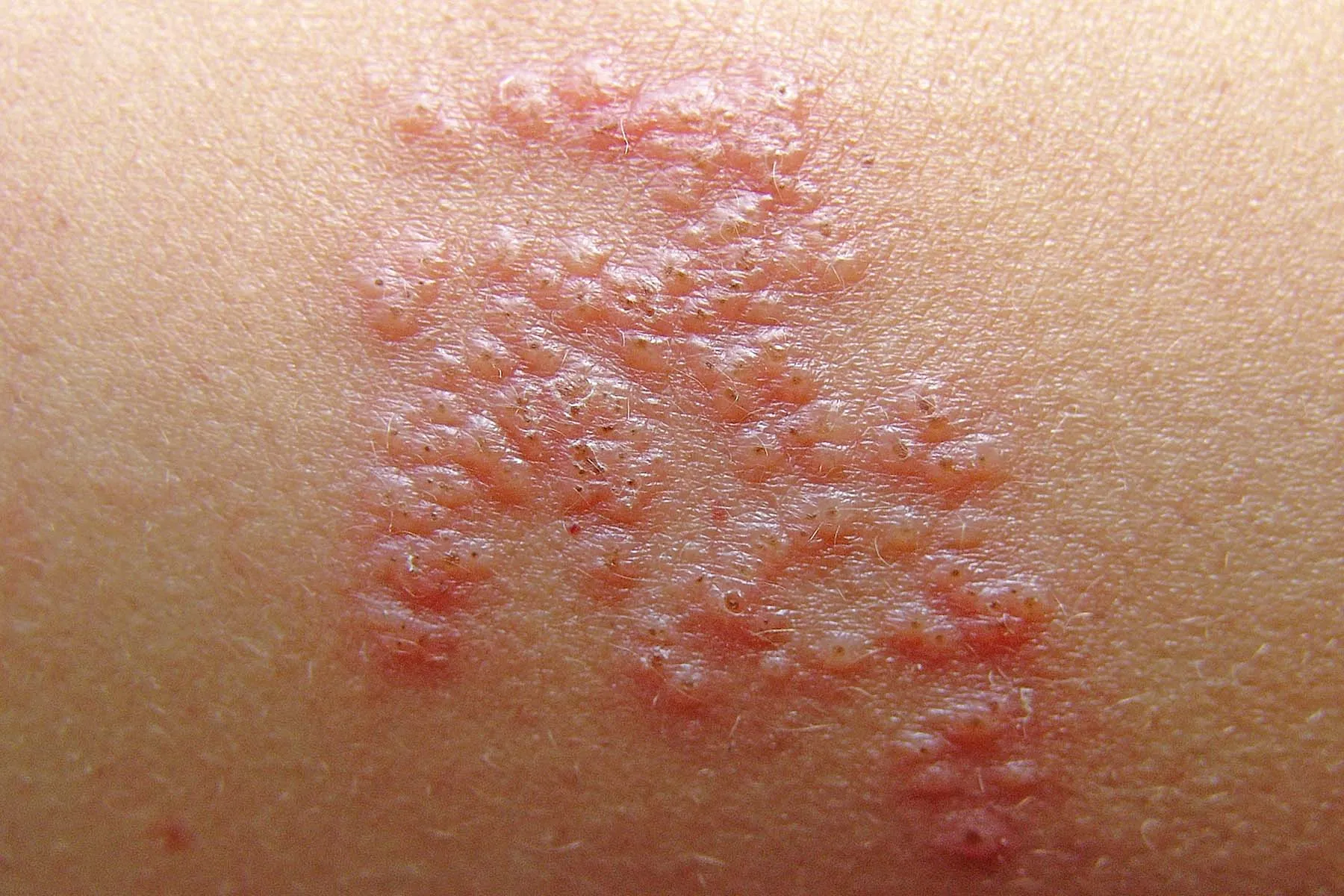
March 20, 2024 — Since its rollout, the Shingrix vaccine has been hailed as a breakthrough in preventing shingles.
But its path to widespread adoption has been strewn with obstacles, from pandemic-related disruptions to insurance complexities. The consequences can be seen in vaccine numbers: Only about 30% of adults eligible for Shingrix have gotten it, according to a 2022 report by the Government Accountability Office.
Health care experts are advocating for its use, emphasizing its unparalleled effectiveness in safeguarding against the painful condition.
“Shingles is a major infectious cause of disability worldwide, and we now have a vaccine that’s showing immediate and potent efficacy against it.,” said Kenneth Koncilja, MD, a specialist from Cleveland Clinic’s Center for Geriatric Medicine. “But there has been a very muddy landscape for a number of reasons since it came out, which complicates things.”
Shingrix, a two-dose shingles vaccine the FDA approved in 2017, was touted as a much more effective alternative to its predecessor, Zostavax.
The CDC estimates that about 1 in every 3 people in the United States will get shingles. Of those, about 10% to 18% will have nerve pain, or postherpetic neuralgia (PHN) – a burning pain in nerves and skin — that can last for years after the rash goes away. Other potential complications include serious eye, heart, and neurological issues, even death.
It can also lead to a nearly 30% increase in risk for cardiovascular events, such a heart attack or stroke, according to research published in the Journal of the American Heart Association.
While Zostavax reduced shingles risk by 51%, Shingrix’s efficacy soared to 97% in people aged 50-69 and 91% in those over 70. It’s also about 90% effective in all age groups in preventing postherpetic neuralgia, compared to 67% withZostavax.
However, in 2017, the concept of a second vaccine dose was daunting for many, compounded by out-of-pocket costs.
“This was well before COVID-19, and many people didn’t even know what the phrase ‘booster’ meant at that point,” Koncilia said.
Steep prices posed a significant obstacle. Numerous Medicare Part D prescription drug plans required a copayment for the shingles vaccine. According to a 2019 report to Congress by the Medicare Payment Advisory Commission, the vaccinecould cost more than $400 for the necessary two doses if a Medicare recipient hadn’t fulfilled their deductible.
That changed in 2023, when Congress abolished cost-sharing for vaccines endorsed by the CDC’s Advisory Committee on Immunization Practices for adults. This policy applies regardless of whether people have drug coverage through Part D or a Medicare Advantage plan, including the shingles vaccine.
“The costs were high and there was a shortage early on,” said Tina Ardon, MD, a family medicine doctor at the Mayo Clinic in Jacksonville, FL. “Some people were only able to get one shot.”
Concerns over potential side effects served as another deterrent. According to the CDC, the vaccine can leave patients with a sore arm, redness, and swelling at the injection site, fatigue, muscle pain, headache, chills, fever, stomach pain, or nausea. These side effects can last for 2 to 3 days.
The COVID pandemic added another layer of complexity, fostering vaccine hesitancy and reducing rates. Among adults with commercial coverage, vaccine administration claims were reportedly 15% lower in December 2020 compared to December 2019, and 62% lower in April 2021 compared to April 2019.
“Does the vaccine work? Yes, it works incredibly well,” said Timothy Brewer, MD, professor of medicine in the Division of Infectious Diseases at the David Geffen School of Medicine at UCLA. “To have a vaccine to use in this population and that works as well as it does is really terrific. I’m hopeful the uptake will improve.”
https://img.lb.wbmdstatic.com/vim/live/webmd/consumer_assets/site_images/articles/health_tools/noncontagious_skin_conditions_to_know_slideshow/1800ss_sciencesource_rm_shingles.jpg
2024-03-20 18:51:48